Why Do Ionic Compounds Conduct Electricity in Solution
Likewise people ask why do ionic compounds not conduct electricity in the solid state. 2 Solutions of ionic compounds and molten ionic compounds can conduct electricity because the ions are free to move around.
Igcse Chemistry 2017 1 56c Understand Why Ionic Compounds Conduct Electricity Only When Molten Or In Aqueous Solution
Why do ionic compounds conduct electricity only when molten or in solution.

. When an ionic substance is molten or dissolved the ions that make it up are free to move within the substance and carry charge through it ie. Why Do Ionic Compounds Conduct Electricity In Aqueous Solution Keywords. Ionic Compounds have ions but they cannot conduct electricity because it lacks free ions.
Why Do Ionic Compounds Conduct Electricity In Aqueous Solution Author. When molten or in solution the ions are free to move. When solid the ions are held in place.
156 Triple only understand why ionic compounds conduct electricity only when molten or in aqueous solution. A solid ionic compound does not conduct electricity because the ions are not free to move. But when an ionic compound dissolves in water to form a solution the constituent ions get separated and they disperse across the solution.
Ionic compounds in the solid state do not conduct electricity because movement of ions in the solid is. This happens because the ions become free and can move from place to place. Ionic compounds only conduct electricity only when molten or in solution.
Why dont ionic compounds conduct electricity in their crystalline form. Ionic compounds are usually formed when metals react with non-metals. Although solid ionic compounds do not conduct electricity because there are no free mobile ions or electrons ionic compounds dissolved in water make an electrically conductive solution.
Ionic compounds can be defined as crystalline solids formed by neatly packed ions of opposite charge. These ions are free to move to conduct electricity. They do not conduct electricity because there are no free electrons.
In Solid State the ions are packed together by strong electrostatic force. Hence covalent compounds do not conduct electricity. When molten or in solution the ions are free to move and carry charge - they are called electrolytes.
Ionic compounds are conductors of electricity when they are in a molten state or aqueous state. This helps in conducting an electric current through the solution. Hence as ions can move it can conduct electricity in molten state.
Ionic compounds can conduct electricity when they are dissolved in water. This is because both processes make their. So ions that conduct electricity are not involved in these compounds.
A An ionic compound consists of ions held by strong forces in the solid state. 12 How are atoms electrically neutral. Although ions are present they cant move because theyre locked in place.
14 Are ionic compounds held together by ionic. 1 Solid ionic compounds dont conduct electricity. Ionic compounds conduct electricity when molten liquid or in aqueous solution dissolved in water because their ions are free to move from place to place.
Why do ionic compounds conduct electricity in aqueous solution Created Date. When solid the ions are not free to move. An aqueous solution of ionic compounds contains ions in mobile from which carry charge and hence help in conduction of electricity.
17 Why do ions form. Therefore they have higher melting and boiling points compared to covalent compounds. Covalent compounds are formed by the sharing of electrons.
Ionic compounds conduct electricity when molten liquid or in an aqueous solution because their ions are free to move from place to place. 11 Why does an ionic compound have to be neutral. However when an ionic compound is solid it exists as a giant ionic lattice where positively charged cations and negatively charged anions are held in a lattice by strong ionic bonds and are not free to move.
In the molten state or in their aqueous solution form ionic compounds contain ions which can move throughout the solution molten solid which helps in conducting electricity. Do ionic compounds conduct electricity when dissolved in water. However in molten state the electrostatic force is a bit low as compared to the solid one which allows the ions to move.
In molten state or dissolved state ionic compounds conduct electricity because they contain charged particles called cations and anions. 10 How do ionic compounds form crystals. Crystalline form means when the ions.
As a result the solution can be made to conduct electricity. In the solid state electrostatic forces hold the ions together in a crystal lattice structure which is in short a 3D interconnected ion network. Ionic compounds have high melting and boiling points because a application of significant amount of energy can break the strong inter-ionic attraction.
Think about this in solid form the outer shells of the elements are filling each other thus neutralizing the substance but when you separate the ions atoms of each element their. This helps in conducting an electric current through the solution. Conduction of electricity is achieved by the movement of charged particles.
No moving ions no electricity. Then why do ionic compounds conduct electricity and covalent compounds do not. Ionic compounds cannot conduct electricity when solid as their ions are held in fixed positions and cannot move.
To form a liquid or dissolved in water to form an aqueous. When an ionic compound dissolves in solution the ions of the molecule dissociate. 15 Can polyatomic ions form ionic compounds.
13 Is an ionic compound always neutral in charge. 16 Are both ionic and molecular compounds neutral. Covalent compounds are formed by the sharing of electrons.
Why or why not. There are plenty of free ions in the molten state or dissolved state which are able to conduct electric current. However if you melt an ionic compound or dissolve it in water these ions are now free to move around.
Wo thats why the conduct electricity.
Why Are Ionic Compound Conductors In A Solution Quora

Ionic Compounds Conduct Electricity In Molten State Why
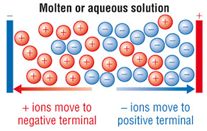
1 56 Triple Only Understand Why Ionic Compounds Conduct Electricity Only When Molten Or In Aqueous Solution Tutormyself Chemistry
No comments for "Why Do Ionic Compounds Conduct Electricity in Solution"
Post a Comment